Fralin Biomedical Research Institute scientists provide new evidence of elusive electrical pathway in the heart
Their study reveals further evidence of the “nuanced interplay” between two prominent cell-to-cell communication pathways that could influence how patients fare during a heart attack.

These days having both a land line and a mobile phone seems like overkill. But Virginia Tech researchers have shown that the heart relies on at least two key communication channels to keep abnormal heart rhythms in check.
In a new study published this summer in the American Journal of Physiology – Heart and Circulatory Physiology, Fralin Biomedical Research Institute at VTC scientists reveal further evidence of the “nuanced interplay” between two prominent cell-to-cell communication pathways that could influence how patients fare during a heart attack.
The research team, led by associate professor Steven Poelzing, discovered they could improve irregular heart rhythms – even when the heart’s blood supply was completely shut off – just by altering concentrations of common electrolytes in the bloodstream. This discovery could have important implications for the prevention and treatment of heart disease, which is the leading cause of death in the United States, according to the Centers for Disease Control and Prevention.
“Millions of Americans take anti-arrhythmic medications or suffer from heart disease. By shedding light on these basic physiological principles, our research could one day help us develop more effective medications and personalized saline solutions to help prevent dangerous arrhythmias,” said Poelzing, who is also an associate professor in the Department of Biomedical Engineering and Mechanics in Virginia Tech’s College of Engineering. “Our goal is to one day help cardiologists identify if a patient could be at higher or lower risk of developing a dangerous arrhythmia based on their blood chemistry.”
Like a phone line, gap junctions are proteins that bridge two adjacent cells. These channels let small molecules, including ions, flow straight from one cell to the next, triggering the ripple of cellular contractions that allow our hearts to beat.
For roughly a century, scientists believed that these protein channels explained how the heart’s electrical impulses passed from cell to cell. But within the past 15 years, mounting evidence has shown that gap junctions aren’t the only mechanism underlying electrical conduction in the heart. When researchers genetically knocked out most of the heart’s gap junctions in mice, they were surprised to find that the test subjects were just as likely to live an ordinary lifespan as their healthy counterparts.
How can hearts to beat if most of the physical ports between their cells are missing? To answer this question, a theory – ephaptic coupling – has re-emerged.
Ephaptic coupling occurs within microscopic spaces wedged between two cell membranes. These pockets, called the perinexus, were first described by Fralin Biomedical Research Institute scientists in 2013 and span just one to two ten-thousandths of a millimeter. For the signaling to work, two cells need to be close enough to sense the electric field generated by their neighboring cell.
“You can think of ephaptic coupling between cells in the context of magnets: When you have two magnets close together they are strongly attracted to each other due to the strength of the magnetic field; similarly, the closer two cells are to one another, the stronger the effect of the electric field will be on each other. But when you pull two magnets apart, you can feel the point where attraction weakens. The same thing happens with electric fields. When the space between cells increases, ephaptic coupling weakens,” said Gregory Hoeker, a research assistant professor in Poelzing’s lab at the Fralin Biomedical Research Institute and the study’s first author.
When blood stops flowing to the heart muscle, its tissues can swell up. This extra fluid between cells pushes the heart cells apart, expanding the width of the perinexus, and preventing ephaptic coupling.
In this new study, Poelzing’s team discovered how the spacing between heart muscle cells changes during a heart attack depends on the specific recipe of electrolytes – calcium, sodium, and potassium – present in the bloodstream. At the organ level, this prevents the heart beats from slowing down and becoming disorganized, which helps normalize the heart rhythm during a heart attack.
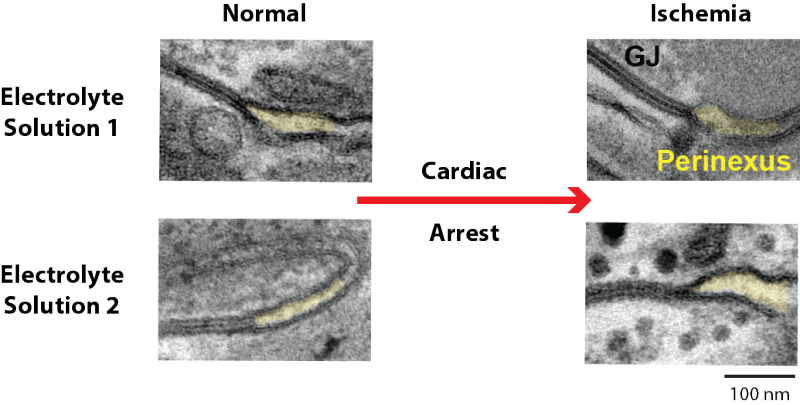
“We’re learning that a patient’s blood salt chemistry before and during a cardiac event is important and could impact their prognosis,” Hoeker said. “The data we have collected so far suggest that these two forms of electrical communication – gap junction coupling and ephaptic coupling – interact in complex ways. Sometimes they work together, sometimes they oppose one another. We believe this balance helps support safe conduction in the heart.”
But there doesn’t seem to be a one-size-fits-all cardioprotective cocktail of electrolytes. One patient may need more calcium and sodium, while another needs less. Small fluctuations in either direction can have a big impact on heart conduction depending on the patient’s baseline blood chemistry. That’s why Poelzing and his team are researching how different saline solutions, ranging from your common intravenous fluid drip bag to the wash that surgeons use during open heart surgeries, impact cardiac function and can contribute to arrhythmias.
The researchers say future experiments will examine how gap junctions and ephaptic coupling interact.
“Our next research step is to take a multilayered approach, using peptide treatments to target gap junctions and different electrolyte fluids to modulate ephaptic coupling, so we can see how these systems work together during an event such as cardiac arrest,” Hoeker said.
This research was funded by a Clinical Research Award in Honor of Mark Josephson and Hein Wellens granted by the Heart Rhythm Society (Hoeker) and the National Institutes of Health, National Heart, Lung, and Blood Institute (Poelzing).